By Madlen Davies , Rosa Furneaux, and Ben Stockton | –
( The Bureau of Investigative Journalism) – As more coronavirus patients were admitted to intensive care, Drayton Hammond, a clinical pharmacist in Chicago, Illinois, frantically tried to order more sedatives. At one point, his hospital was down to a single day’s supply of propofol, one of the main anaesthetics used to keep Covid-19 patients comfortable while they’re connected to ventilators in intensive care. The hospital’s use of this drug had increased fivefold since the pandemic hit, forcing them to ration supplies and use alternative drugs with worrying side effects.
“There’s a grave anxiety,” Hammond said. “You’re accepting that you may be putting people at greater risk for complications.”
Only 40 miles from the hospital is the US headquarters of Fresenius Kabi, a pharmaceutical company which makes the majority of the 12m vials of propofol administered to patients in the US every year. This cloudy white liquid, known as “the milk of amnesia”, is the most widely used intravenous anaesthetic in the world. It has also become a key drug in treating the most severe cases of Covid-19. As it is used to ventilate patients, demand across the world has skyrocketed.
Combining public data, shipping records and dozens of interviews with experts from each stage of the supply chain, the Bureau has begun to piece together how propofol makes its way from the factory to the frontlines of the coronavirus outbreak.
The Bureau has found the production of propofol is concentrated in just a handful of countries. There are five pharmaceutical companies who supply the drug to the US: Fresenius Kabi (which holds nearly 70% of the market), Pfizer, Teva, Sagent and Dr Reddy’s.
All carry out the final stages of manufacture in Europe or Asia. In fact, three of them – Teva, Sagent and Dr Reddy’s – use the same company in Italy to make patient-ready propofol for them, a common set-up in the industry known as contract manufacturing. But experts have warned that depending on just a few overseas companies is precarious, particularly during a crisis.
The drug supply chain is largely hidden from public scrutiny to protect commercial interests. But without transparency, shortages of drugs are often poorly understood, with problems at any stage leading to a patient not being able to receive potentially life-saving treatment.
Changes are needed to ensure a reliable supply of affordable, high quality drugs to patients, experts told the Bureau. They called for greater transparency, better alerts for shortages and rewards for quality manufacturing. Others want manufacturers to diversify their suppliers or to be incentivized to return production to the US.
“I’ve been working on drug shortages since 2001, for almost 20 years, and it is the number one most frustrating thing about working on shortages, how opaque things are,” Erin Fox, a pharmacist and drug shortages expert at the University of Utah hospital, said. “To not even, in some cases, know the name of the company that actually made a drug that I’m going to buy for our hospital system to use for our patients. That is just so frustrating.”
Having a resilient supply of medicines should be factored into the costs of a drug, as it is with most other industries, said Ronald Piervincenzi, chief executive of US Pharmacopeia, an independent scientific non-profit that develops quality standards for medicines.
“The norm is I expect to be able to buy petrol for my car every time I go to buy it,” he said. “They don’t have ‘just in time’ delivery all along [the supply chain], they have big tanks full of gas, they sit around, they wait until the station needs it. And they overfill it because they want to make sure they always have it. It’s built into your price.
“But in medicine, we go right down to the rock bottom price, we leave no room for resilience.”
Step one: Get the active ingredient
Propofol – the”milk of amnesia” Michael Parkin/Folio Art
Propofol gained notoriety as the drug that killed Michael Jackson in 2009. It has, however, been described as the “ideal” anaesthetic because it puts patients to sleep quickly but does not stay in their system too long. Patients can be kept lightly sedated and woken up quickly, free of the “hangover” that the previous most-used anaesthetic induced.
For patients with Covid-19, being treated with propofol means they can be taken off a ventilator and moved from intensive care sooner. This lowers their risk of catching a further infection or suffering side effects associated with longer ICU stays, including delirium and PTSD.
Although Fresenius Kabi’s is the name on the vial of propofol used by many US doctors, production starts at factories owned by three other companies based in Italy, Switzerland and South Carolina. They supply Fresenius Kabi with the active pharmaceutical ingredient, or API, the biologically active part of the drug.
In fact, none of the five brands which manufacture propofol for the US make their own API. According to data from the Food and Drug Administration (FDA), five companies manufacture the API for propofol: the three used by Fresenius Kabi and another two based in India.
It is common for multiple pharmaceutical companies to draw on the same API manufacturers. Even at this very early stage, there is little room for redundancy in the system.
Like many industries, pharmaceutical manufacturing has slowly moved abroad over the past 20 years, typically to India and China. There, labour and raw materials are cheaper, environmental regulations more relaxed, and governments offer tax breaks or other incentives. The FDA estimates more than a quarter of all facilities making the active ingredients for American drugs are based in India and China, but it is not known what volume they contribute.
While this relocation has likely led to lower costs for medicines, experts believe that it is inherently risky to have the world’s supply of drugs consolidated in just a few countries.
The coronavirus crisis has brought this risk to the fore. In March 2020, as the virus took off around the world, India briefly stopped export of raw ingredients, paracetamol and a drug used to treat Covid-19 to conserve domestic supplies. While drug manufacturing has continued while India is on lockdown, transport issues mean that many workers cannot get to the plants. Companies relying on Indian suppliers are rapidly making contingency plans.
Numerous “black swan” events have caused drug shortages in the past decade, including a hurricane that struck Puerto Rico in 2017, a fire in a Chinese factory in 2016 and the travel disruption after the Icelandic volcanic eruption in 2010.
Politics plays its part too. In 2019, there were fears that China could stop exporting medicines when the US imposed tariffs on its goods.
However, the FDA’s 2019 report into the causes of shortages also identified much more everyday structural issues. The complexity of the drug supply chain means that manufacturers face significant logistical and regulatory challenges that make it hard for the market to recover after disruption. There are also few incentives to produce less-profitable drugs and better quality medicines.
The FDA told the Bureau that “even in times of crisis the FDA cannot require companies to start making a drug or make more of a medicine” but added it had contacted manufacturers to identify “potential supply disruptions or shortages in advance”.
Step two: Make the drug
When propofol launched in 1989, doctors were wary of it. Dr David Goodale, who helped launch the drug for AstraZeneca, recalls anaesthetists coming up to him, shaking their fingers in his face and saying that they would not inject milk into their patients. But soon it became America’s most popular sedative.
“Propofol was the first drug in the United States that was not administered in a watery solution,” Goodale told the Bureau. It is this formulation that makes the manufacturing process more complex and costly than tablets.
In order to put people to sleep, propofol has to cross what’s known as the blood–brain barrier. This requires it be formulated as an emulsion, a fine dispersion of small oil droplets suspended in water (milk and mayonnaise are other examples of oil-in-water emulsions). In the case of Fresenius Kabi, the API is shipped to its factories in Austria or Sweden where it is turned into the finished liquid.
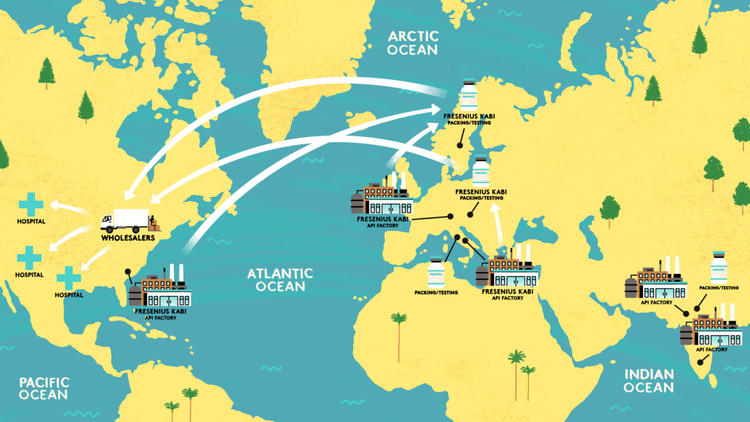
There are a handful of API manufacturers and packing and testing companies identified by the FDA as supplying the US’s propofol. The Bureau has tracked the supply chain for Fresenius Kabi to show how ingredients and medicines cross thousands of miles to reach patients Michael Parkin/Folio Art.
Propofol emulsion is very difficult to make, Goodale said. The propofol is combined with soybean oil, glycerin and egg phospholipids, white tadpole-like molecules with a head that is water soluble and two tails that are fat soluble. The propofol sits at the centre of the droplet, encased by the phospholipids.
Carefully controlled manufacturing conditions are needed to create the emulsion and keep each droplet within a specific size range, Goodale said. If the pH drops, the droplets can coalesce into bigger ones, becoming too big for human capillaries and potentially causing deadly embolisms. Controlled manufacture is also vital for creating propofol that will remain stable for its three-year shelf life.
The entire manufacturing process also needs to be sterile, which requires an expensive plant and precise processes. Because propofol is injected into the body, any contamination with bacteria could cause severe problems. In fact, major infections and deaths occurred in the early 1990s when doctors, not used to using the drug and wanting to conserve supplies, stored partially-used syringes of it in their lockers over the weekend, Goodale said. “That’s like leaving mayonnaise out on your deck in summer and hoping nothing grows in it.”
Goodale and his team had to introduce an antimicrobial, EDTA, into the propofol in order to block growth of any bacteria and fungi. EDTA is still used today in Fresenius Kabi’s propofol.
Step three: Test and pack the drug
The final stage of production involves a series of tests to ensure that the liquid is stable, safe and meets the specifications laid down by the FDA. The vials are sent to another plant to be packaged.
The time-consuming and complex nature of making propofol means there is little economic incentive to join the market. Many companies do not have the capacity to produce it, while others are unwilling to invest time and money when they can make more profit on other medicines.
Making the drug can prove costly in other ways too. Legal action was brought against Teva over its propofol after a number of people undergoing endoscopies in a single clinic in Las Vegas, Nevada in 2007 contracted hepatitis C. A doctor had been “double-dipping” into a “jumbo” vial of propofol to treat multiple patients. In 2011, Teva said it had settled “the vast majority” of cases related to the outbreak, including that of one couple who were initially awarded over $500m by a jury ($356m from Teva). In 2009, the FDA found toxins in Teva’s propofol that could cause “severe fever and even death”.
Teva stopped manufacturing propofol in 2010. In total, nine other companies have stopped making either the API or final dose propofol for the US market in the past 30 years.
There are still manufacturing issues around propofol. In March 2020, just as the global demand skyrocketed, the FDA warned Pfizer – which supplies about 15% of the US’s propofol – of “significant violations” after a plant inspection in India. Pfizer told the Bureau it has a “comprehensive plan in place to address the concerns raised by the FDA” at the plant, which is still operating, and is “committed to ensuring the safety and quality of our medicines”.

Coronavirus has grounded most commercial flights – shipping by boat takes far longer Michael Parkin/Folio Art.
Coronavirus has brought the supply chain to breaking point.
While Fresenius Kabi had propofol stockpiled for emergencies like Covid-19, demand for the drug began to outstrip supply. Speeding up production is extremely hard; making the emulsion takes around 30 days. Pharmaceutical companies are ramping up capacity, but pharmacists won’t see more vials on their shelves soon.
“Doubling production at a moment’s notice is going to be really, really difficult. Impossible, no – I’ve done it – but it takes time,” said John Giannone, a senior director at US Pharmacopeia who worked in the industry for years. “It’s not the kind of thing where you can just turn the crank and say, I’ve got the capacity, let me just start making more.”
Given these constraints, pharmacists are anxious about whether they will receive enough propofol for their patients. “We know it takes a while to manufacture these drugs. We don’t know how much is in the supply chain,” Fox, of the University of Utah Hospital, said.
In some cases, countries have become so concerned about shortages that they have attempted to seize supplies due to be exported. In March, as Italy suffered a major coronavirus outbreak, an Italian commissioner seized a propofol shipment destined for Mexico. Although the shipment was eventually released, it raised fears that other countries could seize and keep critical medicines for themselves. The Bureau contacted the commissioner and received no response.
Doubling the production of propofol relies on the companies at every stage of the supply chain being able to increase their capacity. API plants would have to source more pharmaceutical-grade chemicals and catalysts. The companies making the emulsions would need to buy more glycerin, soybean oil and phospholipids from other companies, as well as more vials and labels, which it may not make in-house.
Each supplier needs to be approved by the pharmaceutical company because any change to quality or process – down to the coating or stoppers used on the vials – could drastically affect how the drug works.
A spokesperson for Fresenius Kabi said it “responded promptly” and its plants were “running at maximum capacity” due to the increased demand. It freed up all suitable resources to make propofol and other drugs to treat Covid-19 patients. “We will, of course, continue to do our utmost to make patient care possible.”
Step four: Get the drug to the patient
After the packaging and safety testing is complete, the finished vials of propofol are shipped from Fresenius Kabi’s plants in Austria or Sweden to America.
Unsurprisingly, the logistics of transporting the API, the vials of propofol and any supplies needed for manufacture have also been drastically affected by the global pandemic. Drugs are commonly transported in the holds of passenger planes, but fewer aircraft are flying, making freight more infrequent and costly.
It is possible to send products by boat, but this takes a lot longer – a matter of weeks rather than days – a real disadvantage to hospitals facing acute shortages. These delays add up at each stage of the supply chain.
Rather than buying drugs directly, most hospitals and pharmacies in the US buy drugs through wholesalers and group purchasing organisations (GPOs), middlemen who aggregate demand across multiple hospitals or pharmacies in order to increase buying power and drive down prices. In the US three wholesalers, McKesson, AmerisourceBergen and Cardinal Health, cover 92% of the market, delivering millions of medicines to hospitals in fleets of branded lorries.
According to the FDA the system has contributed to a “race to the bottom” as the price competition shaves down profit margins, especially in the case of injectables, which are expensive to make. Propofol is a generic drug – no longer under patent – so multiple manufacturers can produce it, and it fetches a much lower price than newer, patented drugs, further squeezing profits.
There is also no incentive to make better quality drugs. “GPOs don’t contract on the quality of products, they only care about the lowest price for a given quantity,” said Dr Rena Conti, a Boston University professor who has studied drug shortages. This leads to the industry’s perception that “GPOs create a race to the bottom on both quality and quantity assurance”.
Industry experts agree that the contracting model is imperfect. During a meeting on shortages organised by the FDA in 2018, Navin Katyal, who oversees Pfizer’s hospital business, said companies could sign a contract with a wholesaler or GPO to make a drug, but still be vulnerable at any time to a “price challenge” from a competitor.
Experts agree that the contracting system for drug supply is deeply flawed Michael Parkin/Folio Art.
Then they have to decide whether to match the lower price, potentially putting their profit in jeopardy, or drop the contract. “It is incredibly difficult for any manufacturer to operate in that environment, with such a low level of certainty or predictability around profitability,” he said.
Companies that cannot match their competitors’ lower prices often decide to stop manufacturing the drug, making the supply chain even more vulnerable.
“Covid-19 is a perfect storm for this system to go awry,” Conti said.
Struggling to supply
100%
Prescriptions for propofol typically fulfilled in Premier Inc hospitals
41%
Propofol prescriptions fulfilled April 1-14, 2020
One GPO, Premier Inc, which buys drugs for nearly 4,000 US hospitals and health systems, has started offering more lucrative contracts for certain cheap drugs in short supply, committing to ordering in volume over a longer period of time to get new companies to enter the market. But propofol is not covered by any of Premier’s new contracts – although it may be in the future, said Premier’s director of advocacy, Soumi Saha.
“One of our big goals is sustainable supply at a sustainable price. There are certain products where Premier actually does not choose the lowest price,” Saha said. “We’re looking for a company that can help ensure a certain level of quality and stability.”
Premier is struggling to buy enough of the drug to meet demand. Typically, hospital pharmacists would be able to fulfil 100% of prescriptions for propofol. According to Premier’s data, in March only 75% of prescriptions were fulfilled. For the first two weeks of April, this figure dropped even lower, to just 41%.
A spokesperson for Sagent said the company was working with its supplier to increase production of propofol “to meet the anticipated increase in demand resulting from the Covid-19 pandemic”. The Bureau contacted Teva and Dr Reddy’s but received no response.
Step five: Make the system better
Ronald Piervincenzi is hopeful that, as shortages have become public, the pandemic provides an opportunity for changes that could make the system more resilient. He said purchasers, whether hospitals, GPOs, donor organisations or national governments, should put pressure on their suppliers to build resilience and quality into their prices.
“I think the start has to be in the demand upstream to say ‘I want to make sure that what you buy for me is resilient’. So if a pharmacy chain has a commitment to their customers and patients to deliver good medicine reliably, they need to put pressure on the purchasing agent and say, ‘I’m willing to give you an extra 5% to give me more resilience in my supply chain,’ and then you start to see it happen.”
Strengthening the supply could involve choosing API suppliers in different countries, rather than concentrated in a manufacturing centre in India or China. “Even if you had a hundred manufacturers of that API in the current circumstances, if all of them were based in Wuhan, China, you’d still have the same problem,” John Giannone said.
Both generic drug makers and API manufacturers should be required to have contingency plans to help ensure a continued supply of medicines, Piervincenzi added. This is the norm for manufacturers of branded medicines, especially medicines under patents, as any shortage would wipe out the whole world’s supply.
The companies create “risk management plans” around their products, including a second plant that could be scaled up quickly and pre-vetted suppliers they could turn to at short notice. Generic companies, admittedly operating on much lower margins, could introduce similar systems. The FDA is considering requiring companies to make such plans.
Risk management plans would mean companies had a second factory to produce ingredients or drugs in the event of a “black swan” disaster Michael Parkin/Folio Art.
Many believe extra transparency could also help the government to mitigate shortages. Until now, companies have only had to report shortages in the finished dose to the FDA. The lists are helpful, said Professor Stephen Schondelmeyer, from the University of Minnesota’s College of Pharmacy, but the reasons for shortages are “very cursory and not really descriptive of what really happened behind the scenes”.
“[The FDA] need to know exactly where it’s coming from, in what quantities, and how much inventory,” he added. At present, pharmacists are not able to tell if there will be a shortage for two weeks or for six months.
There has long been pushback from manufacturers who consider this information to be proprietary. George Zorich, the chief executive of ZedPharma, compares it to buying a meal from a restaurant and asking for the recipe. “You say, ‘That was fantastic. Could you let me know what’s in it?’,” he said. “And they’ll say, ‘No, I’m sorry. That’s our proprietary special recipe.’ I think people just feel there’s no need to know.” Secrecy also allows companies to market their products as if they were made in the US, he said.
Politicians have, however, taken up the issue. As the coronavirus crisis grew, US representatives passed new legislation to mitigate drug shortages. From September companies will be required to report shortages of API as well as the finished medicines, and submit an annual report on the quantity of drugs manufactured or processed at registered facilities.
President Trump is also seeking support for an executive order to force federal agencies to “Buy America”: purchase only domestically made pharmaceutical ingredients, raw materials and medical supplies. But some believe it is unrealistic to ask generic manufacturers with slim profit margins to build expensive API plants in the US. These businesses will either need to be paid more for their products, or receive state aid to incentivise manufacturing in America, said James DeYonker, chief legal and compliance officer at Centrient Pharmaceuticals.
The cost of setting up such a plant could run into hundreds of millions of dollars, with several years wait to get it built and approved for production, he suggested. “So it’s an extremely long time that you have to spend money and lose money before you make anything.”
On top of this expenditure, labour costs in the US or Europe would be double those in India, he said. “Nobody is going to do that for a drug that makes 5% margin,” Yonker added. “So I think a lot of what we see in the media these days is a lot of people saying, ‘All these bad pharma companies, they moved all their manufacturing to India and China’. Well, that’s not really true. I can only speak for generics but we had no choice. It was either that or go out of business.”
In Chicago, with his hospital’s propofol stocks frighteningly low, Hammond continued to put in critical orders. Eventually, more drugs arrived.
But as the supplies came, so did the people who needed them. With more than 100 Covid patients in the ICU, he continues to spend hours each day organising the drugs that will keep them alive, and figuring out how to make hospital stocks last.
“We’re tracking between 20 and 30 different drugs,” he said. “It’s a daily concern. And it will probably be for the foreseeable future.”
Map and illustrations by Michael Parkin for The Bureau of Investigative Journalism
Our reporting on coronavirus is part of our Global Health project, which has a number of funders including the Bill and Melinda Gates Foundation. None of our funders have any influence over the Bureau’s editorial decisions or output.
Via The Bureau of Investigative Journalism
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.




 © 2025 All Rights Reserved
© 2025 All Rights Reserved